The first questions we must answer are: Is all evidence matter? What is matter? How do we classify and collect the matter that matters?
In chemistry as in most sciences, matter is defined as anything that has mass and takes up space. Matter can therefore be weighed and measured and the numbers generated analyzed and compared for the sake of evidence or research. When we measure the matter we will use, it is important to follow the rules of collection and computation that keep the measurements in a form that is capable of comparison. What I mean by this is that you canít weigh an object on a scale capable of only measuring to the 0.1 gram but report it to the 0.001 gram. That would be claiming a degree of precision that does not exist in the measurement. The use of significant figure rules will keep us from making this kind of mistake. We also need to realize that some of the evidence we will collect both in research and in forensic investigation is not in fact matter. Observations, while not a type of matter are of considerable value when investigating. Observations fall under a type of data called qualitative data and are just as important as quantitative (measured or calculated) data.

Qualitative data is the observations investigator makes. While subjective in nature, these observations have value since they often will help an investigator early on decide what evidence to collect or the general direction an investigation should go. Collection of qualitative data might include surveying witnesses or suspects, taking pictures of crime scenes or objects left at a crime scene, etc. Because of the subjective nature of the data, there is more difficulty in comparing the results. We often say that data of this nature can be used to form a consensus but never an absolute answer such as we would get in quantitative data because of the issues with reproducibility.
Quantitative data is the collection of numbers. These numbers could be raw counts like how many people were present at a party or they could be measurements like the mass of an apple. The advantage to quantitative data is that is can be reproduced and is non-subjective in nature. This means if several different investigators attempted the measurement they should all come to the same answer within the standard deviation of the data.

Main Points
Qualitative research involves analysis of data such as words (e.g., from interviews), pictures (e.g., video), or objects (e.g., an artifact).
Quantitative research involves analysis of numerical data.
There are strengths and weaknesses of both qualitative and quantitative investigation.
Overly focusing on the debate of "qualitative versus quantitative" frames the methods in opposition. It is important to focus also on how the techniques can be integrated, such as in mixed methods research. More good can come of developing skills in both realms than debating which method is superior.
We already defined the terms physical and chemical properties of matter in the first lecture, now we will continue with further defining the properties of matter. We can start with the terms intensive properties versus extensive properties. Intensive properties are those properties of matter that do not depend on the amount of the substance that is present. A list of some of the most common intensive properties is shown here:
| Intensive Properties of Matter | ||||
 Color |
Odor |
Luster |
Boiling Point |
Hardness |
Malleability |
Ductility |
Conductivity |
Density |
Pressure |
Gary, Please attach the definitions below to the pictures as rollover or pop-up boxes.
- Color - the wavelengths of light as perceived by the human eye
- Odor - the property of a substance that activates the sense of smell
- Luster - How shiny a substance is.
- Boiling Point - The temperature at which the vapor pressure of a liquid is equal to the pressure on the liquid (generally atmospheric pressure).
- Hardness - How easily a substance can be scratched.
- Malleability - The ability of a substance to be beaten into thin sheets.
- Ductility - The ability of a substance to be drawn into thin wires.
- Conductivity - The ability of a substance to allow the flow of energy or electricity.
- Density - The mass of a substance divided by its volume
- Pressure - the force applied per unit area of a substance
Note that all of these properties (like the example of density above) can be measured or calculated and thus fall under the realm of quantitative data. While you might not think of color as being a number, it in fact can be measured using a spectrometer and the wavelength of the color determined. Odors also are not immediately thought of in terms of numerical data but are in fact gaseous molecules that can be measured by mass spectrometry to yield the percentages of each component compound by mass.
Extensive properties are the more commonly measured physical properties of matter and are dependent on the amount of substance present. Note here that mass and weight are listed as separate items although we often use the two terms interchangeably. As long as we are on Earth, the mass and weight of an object are the same. If we move to the moon or Venus, the mass of an object will remain the same but the weight will change. A person weighing 150 lbs on Earth would only weigh 24.9 lbs on the moon and on Venus, 136 lbs. So although the mass remains 150 lbs the weight changes based on the gravity of the planet. Other measurements like volume and length will be the same no matter where they are taken.
Extensive - Properties that do depend on the amount of matter present:
1) Mass or Weight

Difference between Mass and Weight
| Mass - A measurement of the amount of matter in an object (grams). | Weight - A measurement of the gravitational force of attraction of the earth acting on an object. |
2) Volume:
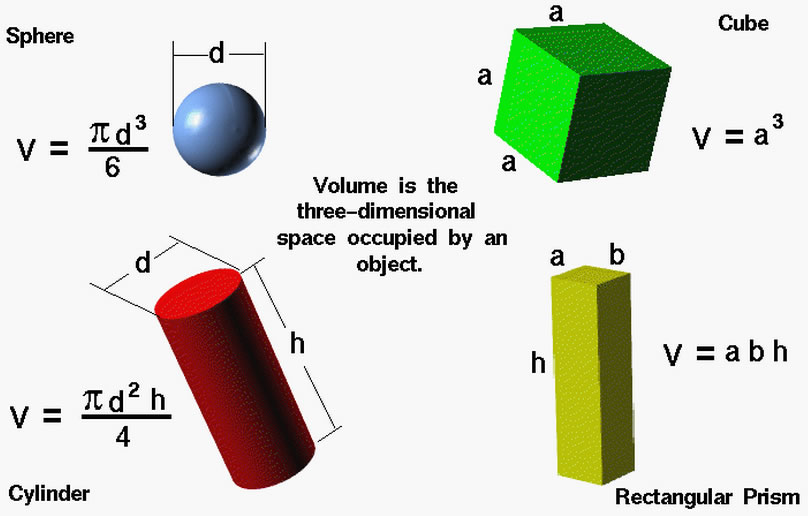
3) Length:
A measure of the amount of distance between the start and end of an object.

http://juniorsteps.global2.vic.edu.au/2013/08/15/measuring-length/








